Eb101 Abeona
In total, 10-15 patients are expected to enroll.

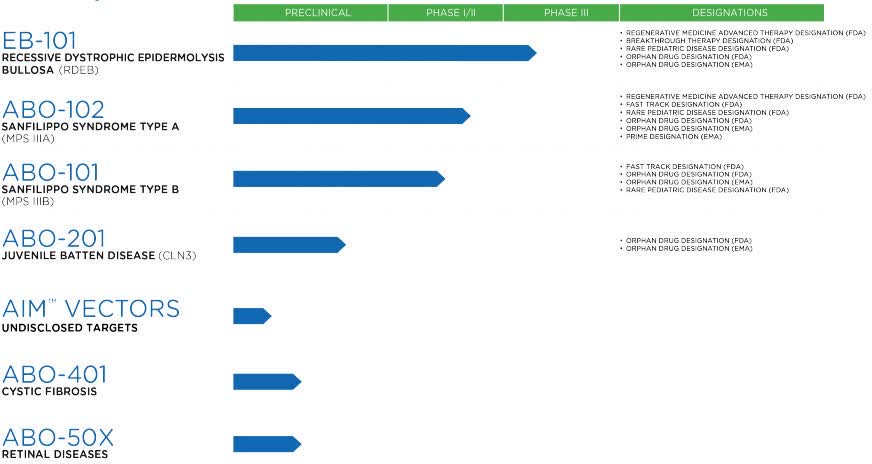
Eb101 abeona. In a Phase 1/2a clinical trial, EB-101 provided durable wound. A single-center randomized clinical trial assessing autologous, gene-corrected cell therapy EB-101 for wound healing in patients with RDEB. In the U.S., Abeona holds Regenerative Medicine Advanced Therapy, Breakthrough Therapy, and Rare Pediatric designations for EB-101 and Orphan Drug designation in both the U.S.
(NASDAQ:ABEO) is about to begin patient enrollment in its pivotal Phase 3 VITAL study of EB-101. Is a clinical-stage biopharmaceutical company developing gene and cell therapies for serious diseases. Is a clinical-stage biopharmaceutical company developing gene and cell therapies for serious diseases.
The enrollment is resuming after a pause in March after a strong need to redirect healthcare resources to COVID-19 patients ascended. In a Phase 1/2a clinical trial, EB-101 provided durable wound healing for RDEB patients lasting 2+ to 5+ years, including for the largest, most challenging wounds that afect the majority of the RDEB population. “The Abeona team has worked diligently to provide a prompt and thorough response to the FDA, enabling us to proceed with our pivotal Phase 3 trial for EB-101,” said João Siffert, M.D., Chief Executive Officer of Abeona.
Abeona is producing EB-101 for the VIITAL TM study at the Elisa Linton Center for Rare Disease Therapies, its fully-functional gene and cell therapy manufacturing facility centrally-located in Cleveland, OH. “The Abeona team has worked diligently to provide a prompt and thorough response to the FDA, enabling us to proceed with our pivotal Phase 3 trial for EB-101,” said João Siffert, M.D., Chief. “The Abeona team has worked diligently to provide a prompt and thorough response to the FDA, enabling us to proceed with our pivotal Phase 3 trial for EB-101,” said João Siffert, M.D., Chief.
“The Abeona team has worked diligently to provide a prompt and thorough response to the FDA, enabling us to proceed with our pivotal Phase 3 trial for EB-101,” said João Siffert, M.D., Chief. ABEO), a fully-integrated leader in gene and cell therapy, today announced that it has received Institutional Review Board (IRB) approval from Stanford University to commence the VIITAL™ study, the. RDEB is a severe form of EB caused by mutations in the COL7A1 gene, which contains.
Treatment effect will be measured as wound healing at 3 months, with follow up to 6 months post treatment, comparing treated versus matched untreated. Abeona Therapeutics has restarted patient enrollment in the VIITAL Phase 3 clinical trial of EB-101, a potential gene-corrected cell therapy for people with recessive dystrophic epidermolysis bullosa (RDEB). Abeona Therapeutics Provides Regulatory Update Ahead of Pivotal Phase 3 Clinical Trial for EB-101 in Recessive Dystrophic Epidermolysis Bullosa Read full article September 23, 19, 8:45 AM.
The Company’s clinical programs include EB-101, its autologous, gene-corrected cell therapy for recessive dystrophic epidermolysis bullosa, as well as ABO-102 and ABO-101, novel AAV9-based. NEW YORK and CLEVELAND, July 08, -- Abeona Therapeutics Inc. “We expect to be a transformational year at Abeona, and we are proud to start it with the initiation of our pivotal Phase 3 study evaluating EB-101 in RDEB,” said João Siffert, M.D., Chief Executive Officer.
Abeona Therapeutics plans to start a Phase 3 trial in 19 testing the cell therapy candidate EB-101 as a treatment for recessive dystrophic epidermolysis bullosa (RDEB). NEW YORK, NY and CLEVELAND, OH, USA I January 13, I Abeona Therapeutics Inc. Abeona Therapeutics Cleared to Initiate Pivotal Phase 3 Clinical Trial Evaluating EB-101 Gene Therapy for Recessive Dystrophic Epidermolysis Bullosa Download as PDF December 09, 19 FDA removes clinical hold;.
Is a clinical stage company developing gene and plasma-based therapies for life-threatening rare genetic diseases. The VIITAL™ Phase 3 study is a multi-center, randomized clinical trial assessing EB-101 in up to 15 RDEB patients, with approximately 30 large, chronic wound sites treated in total. Food and Drug Administration expected in Q4 19.
Company may proceed with VIITAL™ study. Abeona produces EB-101 for the VIITAL TM study at its fully-functional gene and cell therapy manufacturing facility in Cleveland, OH. , a fully-integrated leader in gene and cell therapy, today announced recent updates on its clinical programs, highlighted.
“We look forward to the first patient receiving EB-101 this quarter, setting in motion the final stages of this important program. Abeona will produce EB-101 for the pivotal VIITAL™ study at the Elisa Linton Center for Rare Disease Therapies, its fully-functional gene and cell therapy manufacturing facility, centrally. Abeona produces EB-101 for the VIITALTM study at its fully-functional gene and cell therapy manufacturing facility in Cleveland, OH.
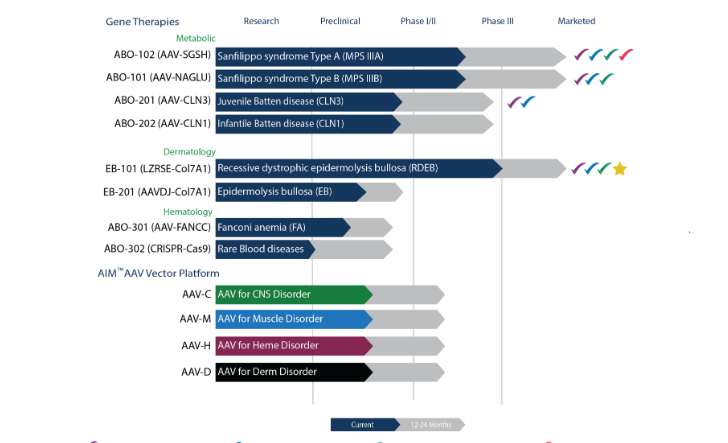
About Abeona Therapeutics Abeona Therapeutics Inc. The Transpher B study, is an open-label, dose-escalation clinical trial evaluating ABO-101 as a treatment for MPS IIIB. Abeona’s EB-101 product is an autologous, ex-vivo gene-corrected cell therapy in which the COL7A1 gene is inserted into a patient’s own skin cells (keratinocytes) for the treatment of the.
Abeona produces EB-101 for the VIITAL TM study at its fully-functional gene and cell therapy manufacturing facility in Cleveland, OH. Abeona’s clinical programs include EB-101, its autologous, gene-corrected cell therapy for recessive dystrophic epidermolysis bullosa in Phase 3 development, as well as ABO-102 and ABO-101. Abeona's lead EB product, EB-101 (gene-corrected skin grafts), is a gene therapy currently in Phase 1/2 clinical trials for the treatment of RDEB patients.
About Abeona Therapeutics Abeona Therapeutics Inc. The Transpher B Study (NCT) is an ongoing, two-year, open-label, dose-escalation, Phase 1/2 global clinical trial assessing ABO-101 for the treatment of patients with Sanfilippo syndrome. After its recent clinical hold by regulators, the Phase 3 VIITAL trial has been given the green light to investigate EB-101, a gene-corrected cell therapy for people with recessive dystrophic epidermolysis bullosa (RDEB).
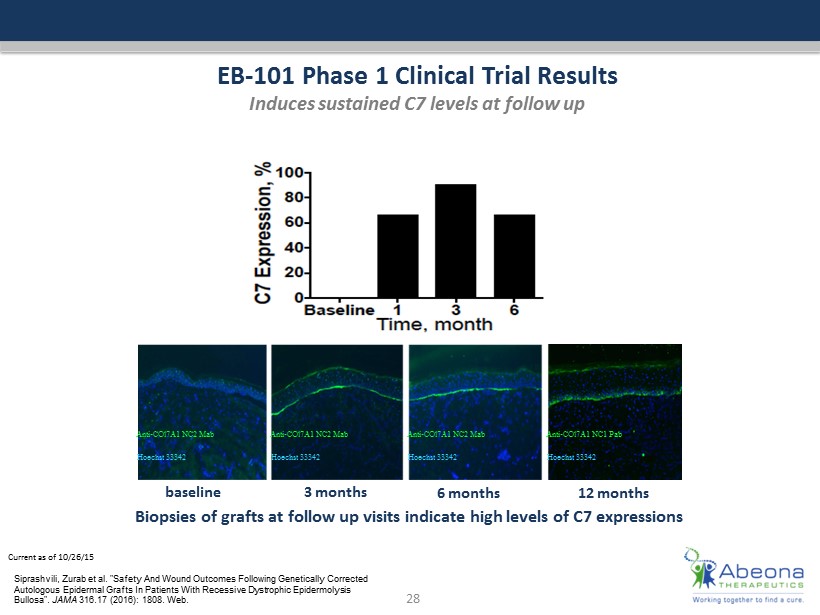
Abeona’s EB-101 product is an autologous, ex-vivo gene-corrected cell therapy in which the COL7A1 gene is inserted into a patient’s own skin cells (keratinocytes) for the treatment of the underlying disease in Recessive Dystrophic Epidermolysis Bullosa. ABEO), a fully-integrated leader in gene and cell therapy, today announced that long-term follow up data from a completed Phase 1/2 study evaluating EB-101 for the treatment of recessive dystrophic epidermolysis bullosa (RDEB) will be presented at the 77th Annual Meeting of the Society for Investigative Dermatology. Abeona is developing EB-101, a skin graph cell therapy aiming to deliver a functional COL7A1 gene to a patient’s own keratinocytes, or cells in the epidermis (outermost skin layer).
Abeona is producing EB-101 for the VIITALTM study at the Elisa Linton Center for Rare Disease Therapies, its fully-functional gene and cell therapy manufacturing facility centrally-located in. Abeona Therapeutics, the company developing EB-101, plans to start VIITAL in early. Abeona has received numerous regulatory designations from the FDA and EMA for its pipeline candidates, including Regenerative Medicine Advanced Therapy designation for two candidates (EB-101 and.
The primary outcome measure is wound healing, comparing treated with untreated wound sites on the same patient. Abeona’s EB-101 product is an autologous, ex-vivo gene-corrected cell therapy in which the COL7A1 gene is inserted into a patient’s own skin cells (keratinocytes) for the treatment of the. Abeona’s clinical programs include EB-101, its autologous, gene-corrected cell therapy for recessive dystrophic epidermolysis bullosa in Phase 3 development, as well as ABO-102 and ABO-101.
Up to 6 (six) EB-101 sheets may be applied to each patient, depending on the area of existing wounds. On the other hand, EB-101, a gene-corrected cell therapy, is viewed as a potential …. Abeona is currently continuing preparations for the pivotal Phase 3 VIITAL TM Study evaluating EB-101 for the treatment of RDEB pending the anticipated receipt of Chemical, Manufacturing and Controls (CMC) clearance from the U.S.
A Phase 1/2 clinical trial (NCT) evaluated EB-101’s safety and effectiveness in wound healing in these patients. | September 9,. Abeona has received numerous regulatory designations from the FDA and EMA for its pipeline candidates and is the only company with Regenerative Medicine Advanced Therapy designation for two candidates (EB-101 and ABO-102).
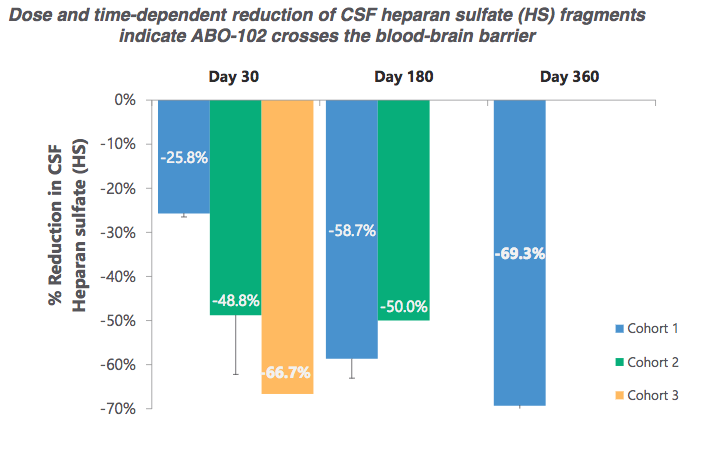
Interim results from the study demonstrate that ABO-101 has been well tolerated to date and shown to improve multiple disease biomarkers providing clear evidence of a biologic effect in patients with MPS IIIB. In a Phase 1/2a clinical trial, EB-101 provided durable wound. ABEO) is a fully-integrated gene and cell therapy company at the forefront of the rapidly-advancing field of genetic medicine.Our expertise across R&D, manufacture, and discovery of novel gene and cell therapies has us uniquely positioned to bring new medicines to patients in need.
EB-101 is a gene therapy, being developed by Abeona therapeutics, for the treatment of recessive dystrophic epidermolysis bullosa (RDEB), an inherited skin disorder caused by defects (mutations) in the COL7A1 gene coding for type VII collagen. Abeona Therapeutics and Taysha Gene Therapies Enter into Licensing and Inventory Purchase Agreements for ABO-2, a Clinical Stage, Novel, One-time Gene Therapy for CLN1 Disease. Abeona’s fully functional, gene and cell therapy GMP manufacturing facility produces EB-101 for the pivotal Phase 3 VIITAL™ study and is capable of clinical and commercial production of AAV.
The multi-center, randomized study, named VITAL, will compare treatment with EB-101 to untreated wounds in the same patient. Abeona's clinical programs include EB-101. A single EB-101 sheet is able to provide healing to a wound area up to approximately 40cm2.

Manufacturing Facility Will Meet Needs Of Cell Gene Trials

News Abeona Therapeutics
2
Eb101 Abeona のギャラリー

Abeona Becomes First Company To Secure Two Rmats

Abeona Therapeutics Opens Ohio Gene And Cell Therapy Manufacturing Facility Pharmaceutical Processing World

Little Abeona Gets Big Setback With Fda Clinical Hold On Pivotal Phase 3 Fiercebiotech

Abeona Restarts Enrollment In Connective Tissue Disorder Study
Abeona Therapeutics String Of Pearls Strategy With Numerous Catalysts And A Lot Of Upside Nasdaq Abeo Seeking Alpha

速递 皮肤病基因疗法获fda突破性疗法认定
Http Pdf Secdatabase Com 1328 18 Pdf

Abeona Therapeutics Reinitiates Enrollment In Eb 101 Pivotal Phase 3 Viital Study In Rdeb After Covid 19 Related Pause And Announces Progress In Patient Enrollment In Mps Iii Studies Nasdaq Abeo

Abeona Therapeutics Cleared To Initiate Pivotal Phase 3 Clinical Trial Evaluating Eb 101 Gene Therapy For Recessive Dystrophic Epidermolysis Bullosa Pcg Advisory

Abeonabio Abeonabio Twitter
Http Www Jefferies Com Cmsfiles Jefferies Com Files Abeona therapeutics inc 1 Pdf

Tv 10k None 10 s

Phase 3 Trial Of Rdeb Therapy Eb 101 To Start In 19 Abeona Therapeutics Says

Abeona Therapeutics Inc Abeo
Abeona Therapeutics Initiates Pivotal Phase 3 Clinical Trial Evaluating Eb 101 Gene Therapy For Recessive Dystrophic Epidermolysis Bullosa Nasdaq Abeo
2

Abeona Restarts Viital Phase 3 Trial Of Eb 101 Presents Positive Phase 1 2 Data

Is A Beat In Store For Abeona Abeo This Earnings Season

Abeona Receives Fda Regenerative Medicine Advanced Therapy Designation For Gene Therapy In Epidermolysis Bullosa The Bio Connection

Abeona Therapeutics Inc 18 Current Report 8 K

Recessive Dystrophic Epidermolysis Bullosa Rdeb Abeona Therapeutics Inc Abeo

Why Abeona Therapeutics Inc And Maybe Sangamo Therapeutics Inc Jumped Higher Today The Motley Fool

Abeona Receives Fda Regenerative Medicine Advanced Therapy Designation For Eb 101 Gene Therapy In Epidermolysis Bullosa Cell Tribune Cell And Gene Therapy News And Research

Abeona Receives Fda Rmat Designation For Gene Corrected Cell Therapy To Treat Epidermolysis Bullosa Global Genes

Abeona S Momentum Continues As Work On Neo Facility Begins

Abeona Therapeutics Abeo Stock News And Breaking Stories Stock Analysis

Orphan Drug For Butterfly Skin Syndrome

Phase 3 Rdeb Trial Testing Abeona S Eb 101 Cell Therapy Begins

Citybizlist New York Abeona Therapeutics Appoints Joao Siffert M D Ceo
Tv 10k None 10 s

Abeona Therapeutics Inc Nasdaq Abeo Says It Is Resuming Patient Enrollment In Its Pivotal Phase 3 Vital Study Of Eb 101 After Covid 19 Related Pause Biopharmajournal

Abeona Therapeutics Leveraging Innovations In Gene And Cell Therapy To Forge Ahead Avise Analytics

Recent Publication About Phase 1 2a Clinical Trial Eb101 Abeona Eb Research
Abeona Therapeutics Initiates Pivotal Phase 3 Clinical Trial Evaluating Eb 101 Gene Therapy For Recessive Dystrophic Epidermolysis Bullosa Nasdaq Abeo
Form 8 K Abeona Therapeutics Inc For Feb 16
Http Pdf Secdatabase Com 1328 18 Pdf

Struggling Abeona Loses President Chief Scientist Fiercebiotech
Investors Abeonatherapeutics Com All Sec Filings Content 19 19 Pdf

Abeona Therapeutics Gets Fda Clearance To Proceed With Phase 3 Clinical Trial Of Its Eb 101 Cell Therapy
2

Fda Puts Clinical Hold On Abeona S Phase Iii Study Of Eb 101

Abeona Treats First Patient In Pivotal Gene Therapy Study

Abeona Therapeutics Youtube
Abeona Enrols 5th Patient In Gene Therapy Epidermolysis Bullosa Trial

Abeona Therapeutics Inc Nasdaq Abeo Stock More Than Tripled In 17

Abeonabio Abeonabio Twitter

News About Abeona

Abeona Therapeutics Youtube

Abeona Therapeutics Don T Be Afraid To Buy The Hype Small Cap Stocks

Fondazione Registro Epidermolisi Bollosa Studio Eb101 Di Abeona Therapeutics
Why Abeona Therapeutics Jumped 14 On Wednesday Business Markets And Stocks News Madison Com

Form 8 K Abeona Therapeutics Inc For Feb 16

Abeona Therapeutics Receives Fda Orphan Designation For Gene Therapy

Recessive Dystrophic Epidermolysis Bullosa Rdeb Abeona Therapeutics Inc Abeo
2

Recent Publication About Phase 1 2a Clinical Trial Eb101 Abeona Eb Research

Neotrans Biotech Firm Abeona To Expand Cleveland Production Facilities

Phase 3 Trial Of Rdeb Therapy Eb 101 To Start In 19 Abeona Therapeutics Says

Zdeymii Fjddim

Abeona Breaks Ground On Gene Therapy Manufacturing Facility In Cleveland Global Genes

Abeona Therapeutics Inc 18 Current Report 8 K

Gene Therapies Go Skin Deep To Tackle Epidermolysis Bullosa Evaluate
Digital Journal A Global Digital Media Network

Abeona Therapeutics Announces Positive Interim Data From The Abo 102 Phase 1 2 Gene Therapy Clinical Trial In Mps Iiia Specialty Pharma Journal

Abeona Therapeutics Abeo Stock News And Breaking Stories Stock Analysis

Abeona Therapeutics Inc Abeo

Abeona Therapeutics Cleared To Initiate Pivotal Phase 3 Clinical Trial Evaluating Eb 101 Gene Therapy For Recessive Dystrophic Epidermolysis Bullosa
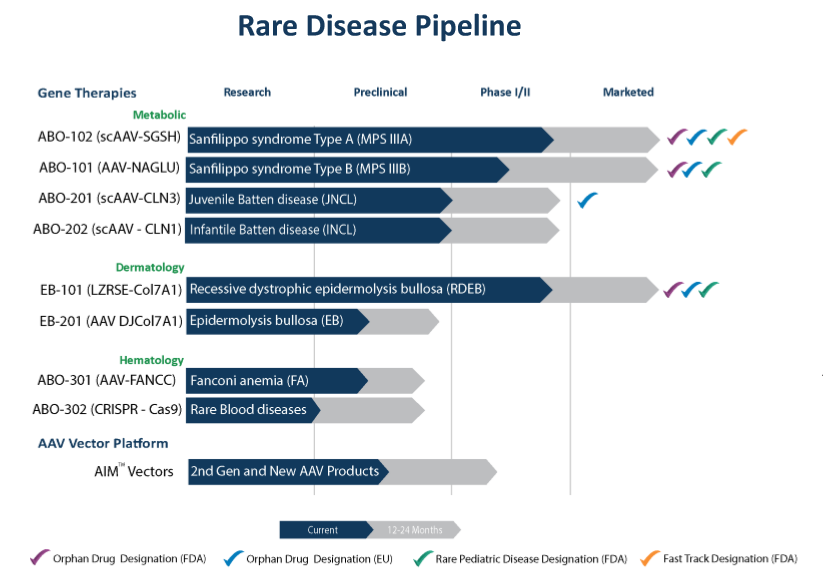
Abeona Therapeutics String Of Pearls Strategy With Numerous Catalysts And A Lot Of Upside Nasdaq Abeo Seeking Alpha

Abeona Therapeutics Inc 18 Current Report 8 K

Abeonabio Abeonabio Twitter

Abeona Therapeutics Inc 18 Current Report 8 K

Here S Why Abeona Therapeutics Fell As Much As 43 8 Today The Motley Fool

Abeona Therapeutics Inc 18 Current Report 8 K
Cash Strapped Abeona S Stock Skyrockets As Gene Therapy Company Explores Strategic Options
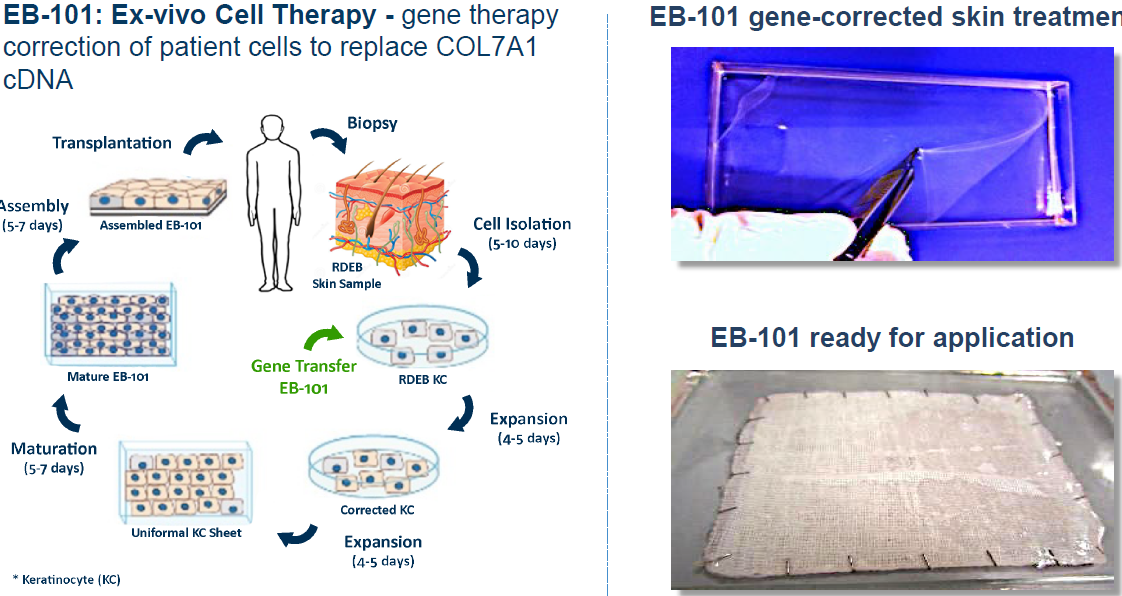
Abeona Therapeutics A Potential Leader In Gene Therapy Nasdaq Abeo Seeking Alpha

Recessive Dystrophic Epidermolysis Bullosa
2

Abeonabio Abeonabio Twitter
2

News About Abeona

Abeona Therapeutics Inc Posts Facebook

Abeonabio Abeonabio Twitter

Abeona Alert Bragar Eagel Squire P C Announces That A Class Action Lawsuit Has Been Filed Against Abeona Therapeutics Inc And Encourages Investors To Contact The Firm Business Wire

Abeona Therapeutics Announces Publication Of Positive Long Term Data From Phase 1 2a Clinical Trial Evaluating Eb 101 Gene Therapy For Recessive Dystrophic Epidermolysis Bullosa Pcg Advisory

Science Abeona Therapeutics Inc Abeo

Fda Halts Abeona S Eb 101 Trial For Rdeb Due To Transport Concerns

Fda Places Hold On Abeona Ahead Of Pivotal Trial Of Cell Therapy For Rdeb Global Genes

Abeona Opens New Manufacturing Facility For Its Gene And Cell Therapies

Abeona Trial Setback Sends Shares Downwards
Abeona Therapeutics Rallies On B Riley S Buy Rating Nasdaq Abeo Seeking Alpha

Abeona Therapeutics Abeo Stock News And Breaking Stories Stock Analysis
18 Could Be Another Solid Year For Abeona Therapeutics Nasdaq Abeo Seeking Alpha

Abeona Treats First Patient In Pivotal Gene Therapy Study

Abeona Ejects Ceo Carsten Thiel For Personal Misconduct Fiercebiotech
18 Could Be Another Solid Year For Abeona Therapeutics Nasdaq Abeo Seeking Alpha

Science Abeona Therapeutics Inc Abeo

Form 8 K Abeona Therapeutics Inc For Dec 06



